The Analytical Process
Discover Neotron's analytical process, the step-by-step quality assurance programme that sets us apart and enables us to deliver reliable and trusted analytical results.
QUALITY AND PRECISION
“Without a defined quality assurance programme all analytical results must be suspect.”
(Harnly and Wolf, 1984)
The Neotron quality assurance program is an integrated system developed at different levels of the organization, all phases of the analysis process are covered by one or more levels of quality control.
The quality assurance program is a set of procedures aiming to guarantee reliable results and minimizing the possibility of analytical mistakes.
DIFFERENT LEVELS OF QUALITY ASSURANCE PROGRAMME
Level 1: Laboratory “horizontal” quality control program (no method-specific)
Level 1 “horizontal quality control program” is intended for all the analytical activity without distinction based on the analytical method in object.
Main “Level 1” Quality control procedures
- Samples registration phase
- Analytical workflow traceability (barcode project).
Traceability along the whole analytical process avoids any risk of mixingup samples and represents a precious tool for tracking and controlling the sample flow. - Equipment performance test.
Performances of instrumental systems are regularly checked by injecting a reference material solution several times. The area and the retention time of analyte has to be stable within a defined tolerance. The performances control is evaluated by acceptability criteria defined for every type of instrument. - Reference materials management.
The wrong management or the poor quality of reference materials is one of the main source of errors in analytical activity.
Neotron analytical determination are performed using certified reference materials stored in a dedicated room with restricted access (automatic locked door). Refrigerators temperature is constantly monitored.
All reference materials are registered in a database containing the following information:
- NAME and CAS Number
- Unique code
- Expiring Date
- Storage temperature
- Lot number
Level 2: Analytical method validation / accreditation
Before introduction in routine use analytical methods are first validated and “core-business” methods are accredited.
1. Analytical approach choice
Decision of use/not use instruments and kind of instruments on the basis of method fit for purpose
2. Definition of the analytical protocol and feasibility test
Definition of all the analytical phases: sample preparation, extraction, purification, detection parameters. Feasibility tests to see if the method works properly
3. Method validation
Series of tests repetitions and results statistical treatment to evaluate the method performances
Method validation > Performance > Validation parameters
Linearity Selectivity Limit of quantification/detection Precision Accuracy Uncertainty
Examples of guidelines used for analysis methods validation:
- ISO/IEC 17025
- USP <1225>
- ICH Q2 (R1)
- European guidelines (e.g. 2002/657/EC)
- SANTE/11813/2017
4. Acceptance criteria
Level 2 is “vertical” because it is method-specific and matrix-specific.
Level 3: Routine activity internal quality control
Quality must be assured during day-by-day analytical activity.
For each analytical batch we can have different internal quality controls (IQC).
IQC are realized with respect to criteria specifically defined in order to guarantee that routine analysis are carried out respecting our quality standards and specifications.
| Parameter | IQC (Internal Quality Control) |
Criteria (defined by the method validation, criteria reported below are examples) |
|---|---|---|
| Recovery | spiked sample / QC sample | 70% – 120% |
| Linearity | Calibration curve | R2 > 0.995 |
| Sensibility | Calibration curve | LoD calibration point S/N > 6 |
| Operator performances | Blind control sample | Accuracy and reproducibilty |
| Interference/Contamination | Reagents blank | Absence of interfering signal or contaminated reagent |
| Matrix-effect | Solvent calibration vs matrix calibration | Evaluation of the true recovery |
Level 4: Inter-laboratory control (proficiency test)
Proficiency test (P-test) is an efficient and important tool to prove the competence of laboratory and the reliability of the analytical results.
P-test is an inter-laboratory activity in which different laboratories perform the test of the same analyte/s in the same sample to evaluate and compare laboratory performance.
Neotron takes part in many “Proficiency Tests” for different kinds of analyses made on plenty of matrixes.
In the last years Neotron has been involved in around 150 P-test every year: providers, accrediated in conformity with Regulation ISO/IEC 17043:2010 “Conformity assessment — General requirements for proficiency testing, to which Neotron usually refers are:
- FAPAS (UK): around 70 P-test for year
- LGC (UK) for microbiology (UK): around 50 P-test for year
- Others: (COI – olive oil), BIPEA (Filth test, nutritional analysis, contaminants), QS (Pesticides and Dioxins -PCBs)
- GAFTA (Analysis in Grain and Feed)
- FOSFA (UK): Ptest related to specific analyses on oil and oil seeds
- Health Ministry Norway: Ptest DIOXIN e PCB
- PROOF-ACS for contaminants
- DRRR for total migration analysis, specific, and MOSH -MOAH
This activity of Quality Assurance is realized according to an Annual Plan, which can be shared with customers.
In case the customer is interested to know the P-Test results regarding a certain method, these results can be communicated after request.
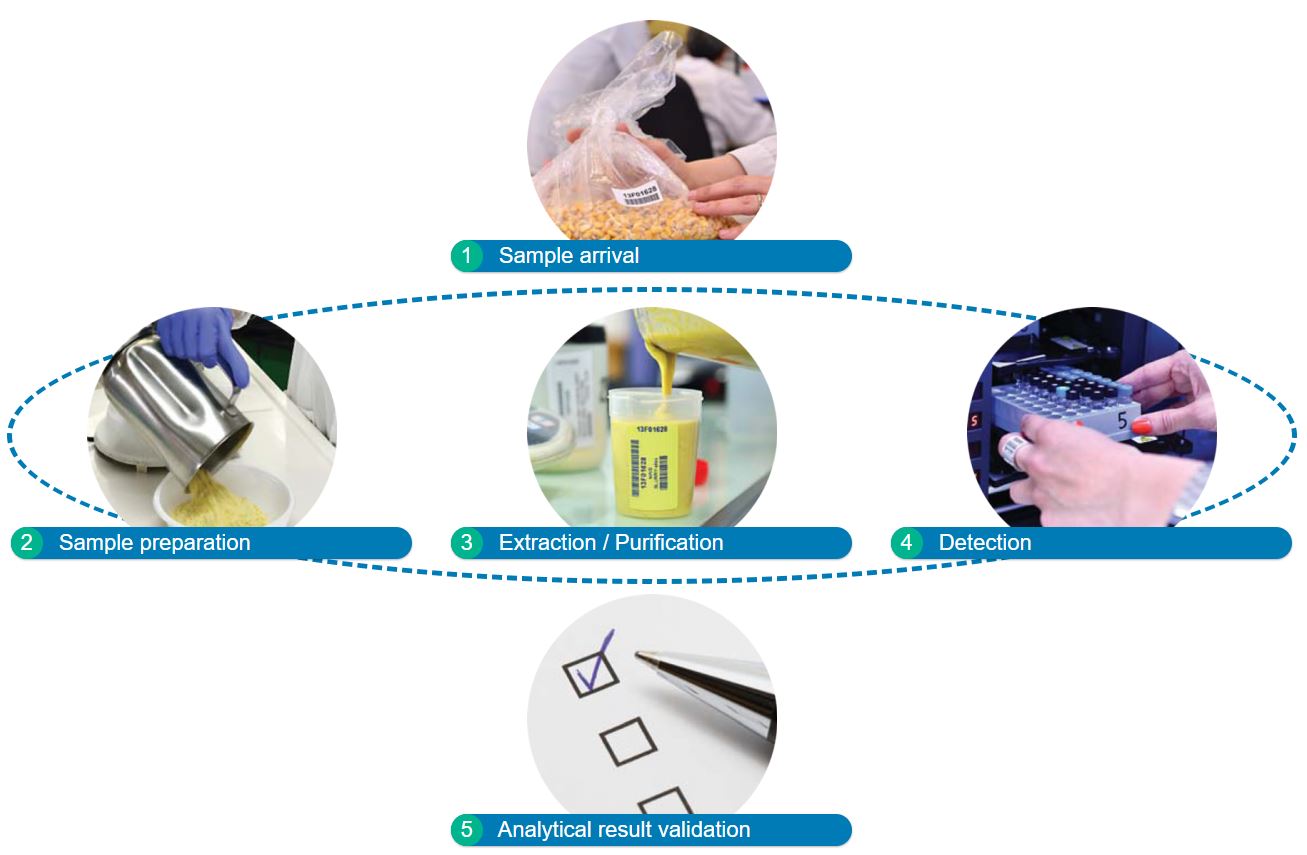
THE ANALYTIC PROCESS
The quality assurance program covers each single analytical activity step with one or more levels.
How can we help you?
To discover how our services can benefit you, or to receive a quotation, please contact us.
Our Company
About Neotron
Neotron is a global leader in testing, analysis, and consulting services on food products, supplements, materials in contact with food (FCM), cosmetics, and pharmaceutical products.
Learn More
