In Vivo Safety Testing
According to the Regulation EC 1223/2009 all cosmetic products must be formulated and marketed in such a way as not to cause harm to people's health.
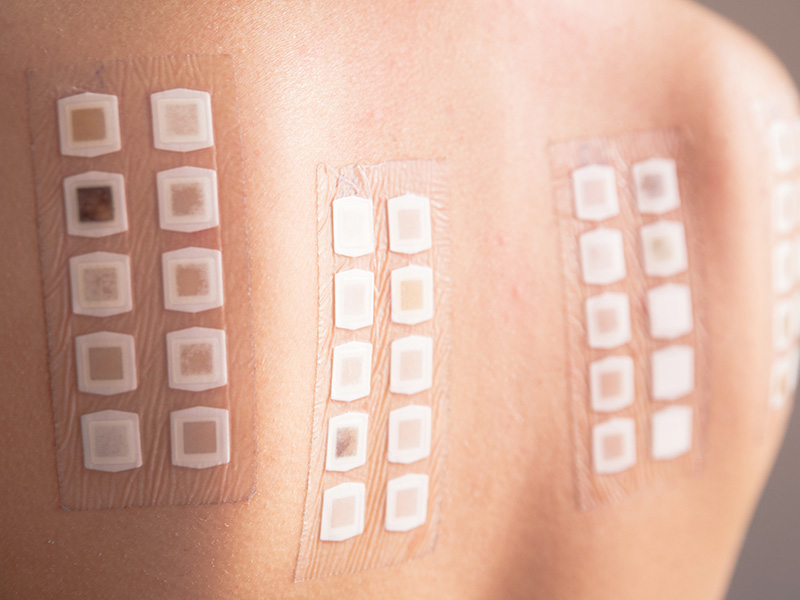
Patch Test 48h
In Neotron can carry out an assessment of the compatibility of the cosmetic with the skin to guarantee the safety of application to the final consumers. Through the patch test 48H, it is possible to evaluate the skin tolerability of the products by identifying and classifying their potencial irritancy.
The study is conducted under the supervision of a dermatologist on a panel of healthy volunteers recruited according to inclusion / exclusion criteria, in accordance with international regulatory guidelines and in full compliance with the ethical principles in the Declaration of Helsinki.
Skin Tolerability Test
Skin compatibility is assessed on a panel of volunteers who apply the cosmetic product under normal conditions of use.
The skin condition is clinically assessed by a dermatologist before and after the treatment and the volunteers are asked to fill out a short questionnaire to highlight any undesirable effects encountered during the test.

Request an Analysis
To learn more about our services, or to request an analysis for your business, please don't hesitate to contact us.
How can we help you?
To discover how our services can benefit you, or to receive a quotation, please contact us.

Services
Cosmetics
Neotron supports cosmetic companies throughout the product control and development process, offering highly specialized services for the assessment of safety and compliance with national and international standards of both cosmetic raw materials and finished products.
Learn More
